SIRT1, an antiinflammatory and antiaging protein, is decreased in lungs of patients with chronic obstructive pulmonary disease
- PMID: 18174544
- PMCID: PMC2292827
- DOI: 10.1164/rccm.200708-1269OC
SIRT1, an antiinflammatory and antiaging protein, is decreased in lungs of patients with chronic obstructive pulmonary disease (VSports app下载)
Abstract
Rationale: Abnormal inflammation and accelerated decline in lung function occur in patients with chronic obstructive pulmonary disease (COPD). Human sirtuin (SIRT1), an antiaging and antiinflammatory protein, is a metabolic NAD(+)-dependent protein/histone deacetylase that regulates proinflammatory mediators by deacetylating histone and nonhistone proteins. VSports手机版.
Objectives: To determine the expression of SIRT1 in lungs of smokers and patients with COPD, and to elucidate the regulation of SIRT1 in response to cigarette smoke in macrophages, and its impact on nuclear factor (NF)-kappaB regulation V体育安卓版. .
Methods: SIRT1 and NF-kappaB levels were assessed in lung samples of nonsmokers, smokers, and patients with COPD. Human monocyte-macrophage cells (MonoMac6) were treated with cigarette smoke extract (CSE) to determine the mechanism of CSE-mediated regulation of SIRT1 and its involvement in RelA/p65 regulation and IL-8 release V体育ios版. .
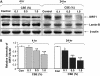
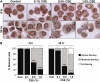
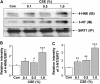
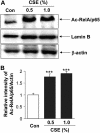
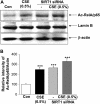
Measurements and main results: Peripheral lungs of smokers and patients with COPD showed decreased levels of nuclear SIRT1, as compared with nonsmokers, associated with its post-translational modifications (formation of nitrotyrosine and aldehyde carbonyl adducts) VSports最新版本. Treatment of MonoMac6 cells with CSE showed decreased levels of SIRT1 associated with increased acetylation of RelA/p65 NF-kappaB. Mutation or knockdown of SIRT1 resulted in increased acetylation of nuclear RelA/p65 and IL-8 release, whereas overexpression of SIRT1 decreased IL-8 release in response to CSE treatment in MonoMac6 cells. .
Conclusions: SIRT1 levels were reduced in macrophages and lungs of smokers and patients with COPD due to its post-translational modifications by cigarette smoke-derived reactive components, leading to increased acetylation of RelA/p65 V体育平台登录. Thus, SIRT1 plays a pivotal role in regulation of NF-kappaB-dependent proinflammatory mediators in lungs of smokers and patients with COPD. .
Figures





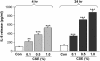
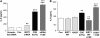
References (VSports)
-
- Pauwels RA, Buist AS, Calverley PM, Jenkins CR, Hurd SS. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) workshop summary. Am J Respir Crit Care Med 2001;163:1256–1276. - V体育ios版 - PubMed
-
- Murray CJ, Lopez AD. Mortality by cause for eight regions of the world: Global Burden of Disease Study. Lancet 1997;349:1269–1276. - PubMed
-
- Vogelmeier C, Bals R. Chronic obstructive pulmonary disease and premature aging. Am J Respir Crit Care Med 2007;175:1217–1218. - V体育官网入口 - PubMed
-
- Tuder RM. Aging and cigarette smoke: fueling the fire. Am J Respir Crit Care Med 2006;174:490–491. - "V体育平台登录" PubMed
-
- Barnes PJ. Chronic obstructive pulmonary disease. N Engl J Med 2000;343:269–280. - PubMed
Publication types
"V体育2025版" MeSH terms
- Actions (VSports注册入口)
- "V体育平台登录" Actions
- VSports app下载 - Actions
- V体育安卓版 - Actions
- Actions (VSports)
- "VSports最新版本" Actions
- "VSports" Actions
- "VSports" Actions
- Actions (VSports注册入口)
- "VSports" Actions
- Actions (V体育安卓版)
- Actions (VSports在线直播)
- Actions (VSports在线直播)
"V体育2025版" Substances
- "V体育官网" Actions
- V体育官网入口 - Actions
- VSports注册入口 - Actions
V体育官网入口 - Grants and funding
LinkOut - more resources
"V体育2025版" Full Text Sources
Other Literature Sources (VSports手机版)
Medical
Molecular Biology Databases

