Antiribosomal-P autoantibodies from psychiatric lupus target a novel neuronal surface protein causing calcium influx and apoptosis (VSports手机版)
- PMID: 18056288
- PMCID: PMC2150977
- DOI: 10.1084/jem.20071285
Antiribosomal-P autoantibodies from psychiatric lupus target a novel neuronal surface protein causing calcium influx and apoptosis
Abstract
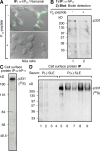
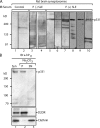
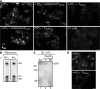
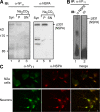
The interesting observation was made 20 years ago that psychotic manifestations in patients with systemic lupus erythematosus are associated with the production of antiribosomal-P protein (anti-P) autoantibodies. Since then, the pathogenic role of anti-P antibodies has attracted considerable attention, giving rise to long-term controversies as evidence has either contradicted or confirmed their clinical association with lupus psychosis. Furthermore, a plausible mechanism supporting an anti-P-mediated neuronal dysfunction is still lacking. We show that anti-P antibodies recognize a new integral membrane protein of the neuronal cell surface VSports手机版. In the brain, this neuronal surface P antigen (NSPA) is preferentially distributed in areas involved in memory, cognition, and emotion. When added to brain cellular cultures, anti-P antibodies caused a rapid and sustained increase in calcium influx in neurons, resulting in apoptotic cell death. In contrast, astrocytes, which do not express NSPA, were not affected. Injection of anti-P antibodies into the brain of living rats also triggered neuronal death by apoptosis. These results demonstrate a neuropathogenic potential of anti-P antibodies and contribute a mechanistic basis for psychiatric lupus. They also provide a molecular target for future exploration of this and other psychiatric diseases. .
Figures



References
-
- Sherer, Y., A. Gorstein, M.J. Fritzler, and Y. Shoenfeld. 2004. Autoantibody explosion in systemic lupus erythematosus: more than 100 different antibodies found in SLE patients. Semin. Arthritis Rheum. 34:501–537. - PubMed
-
- West, S.G. 2007. The nervous system. In Dubois' Lupus Erythematosus. Seventh edition. D.J. Wallace and B.H. Hahn, editors. Lippincott Williams & Wilkins, Philadelphia. 707–746.
-
- Hanly, J.G., A. Kuznetsova, and J.D. Fisk. 2007. Psychopathology of lupus and neuroimaging. In Dubois' Lupus Erythematosus. Seventh edition. D.J. Wallace and B.H. Hahn, editors. Lippincott Williams & Wilkins, Philadelphia. 747–774.
-
- Hanly, J.G., M.B. Urowitz, J. Sanchez-Guerrero, S.C. Bae, C. Gordon, D.J. Wallace, D. Isenberg, G.S. Alarcon, A. Clarke, S. Bernatsky, et al. 2007. Neuropsychiatric events at the time of diagnosis of systemic lupus erythematosus: an international inception cohort study. Arthritis Rheum. 56:265–273. - PubMed
-
- Karassa, F.B., A. Afeltra, A. Ambrozic, D.M. Chang, F. De Keyser, A. Doria, M. Galeazzi, S. Hirohata, I.E. Hoffman, M. Inanc, et al. 2006. Accuracy of anti-ribosomal P protein antibody testing for the diagnosis of neuropsychiatric systemic lupus erythematosus: an international meta-analysis. Arthritis Rheum. 54:312–324. - PubMed
Publication types (VSports注册入口)
- "VSports最新版本" Actions
V体育平台登录 - MeSH terms
- Actions (VSports注册入口)
- VSports注册入口 - Actions
- Actions (VSports在线直播)
- V体育ios版 - Actions
- "VSports" Actions
- VSports在线直播 - Actions
- "V体育安卓版" Actions
- V体育安卓版 - Actions
- "V体育官网" Actions
- "VSports手机版" Actions
Substances
- Actions (V体育2025版)
- "V体育ios版" Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources (VSports手机版)

