"VSports在线直播" Control of the SOST bone enhancer by PTH using MEF2 transcription factors
- PMID: 17696759
- PMCID: PMC2882185
- DOI: 10.1359/jbmr.070804
Control of the SOST bone enhancer by PTH using MEF2 transcription factors
Abstract
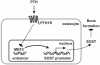
Expression of the osteocyte-derived bone formation inhibitor sclerostin in adult bone requires a distant enhancer VSports手机版. We show that MEF2 transcription factors control this enhancer and mediate inhibition of sclerostin expression by PTH. .
Introduction: Sclerostin encoded by the SOST gene is a key regulator of bone formation. Lack of SOST expression is the cause for the progressive bone overgrowth disorders sclerosteosis and Van Buchem disease. We have previously identified a distant enhancer within the 52-kb Van Buchem disease deletion downstream of the SOST gene that is essential for its expression in adult bone. Furthermore, we and others have reported that SOST expression is suppressed by PTH V体育安卓版. The aim of this study was to identify transcription factors involved in SOST bone enhancer activity and mediating PTH responsiveness. .
Materials and methods: Regulation of the SOST enhancer and promoter was studied by luciferase reporter gene assays. Transcription factor binding sites were mapped by footprint analysis and functional mutation analyses using transient transfections of osteoblast-like UMR-106 cells that exhibit endogenous SOST expression. Specific transcription factor binding was predicted by sequence analysis and shown by gel retardation assays and antibody-induced supershifts. Expression of myocyte enhancer factors 2 (MEF2) was detected by in situ hybridization, quantitative RT-PCR (qPCR), and immunohistochemistry. The role of MEF2s in SOST expression was assessed by reporter gene assays and siRNA-mediated RNA knockdown. V体育ios版.
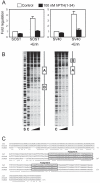
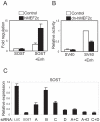
Results: PTH completely suppressed the transcriptional activity of the SOST bone enhancer but did not affect the SOST promoter. A MEF2 response element was identified in the bone enhancer. It was essential for transcriptional activation, bound MEF2 transcription factors, and mediated PTH responsiveness VSports最新版本. Expression of MEF2s in bone was shown by qPCR, in situ hybridization, and immunohistochemistry. MEF2s and sclerostin co-localized in osteocytes. Enhancer activity was stimulated by MEF2C overexpression and inhibited by co-expression of a dominant negative MEF2C mutant. Finally, siRNA-mediated knockdown of MEF2A, C, and D suppressed endogenous SOST expression in UMR-106 osteoblast-like cells. .
Conclusions: These data strongly suggest that SOST expression in osteocytes of adult bone and its inhibition by PTH is mediated by MEF2A, C, and D transcription factors controlling the SOST bone enhancer V体育平台登录. Hence, MEF2s are implicated in the regulation of adult bone mass. .
Figures

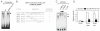
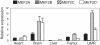

References
-
- Janssens K, Van Hul W. Molecular genetics of too much bone. Hum Mol Genet. 2002;11:2385–2393. - PubMed (VSports在线直播)
-
- van Bezooijen RL, ten Dijke P, Papapoulos SE, Lowik CW. SOST/sclerostin, an osteocyte-derived negative regulator of bone formation. Cytokine Growth Factor Rev. 2005;16:319–327. - "VSports" PubMed
-
- Balemans W, Ebeling M, Patel N, Van Hul E, Olson P, Dioszegi M, Lacza C, Wuyts W, Van Den EJ, Willems P, Paes-Alves AF, Hill S, Bueno M, Ramos FJ, Tacconi P, Dikkers FG, Stratakis C, Lindpaintner K, Vickery B, Foernzler D, Van Hul W. Increased bone density in sclerosteosis is due to the deficiency of a novel secreted protein (SOST) Hum Mol Genet. 2001;10:537–543. - PubMed
-
- Balemans W, Patel N, Ebeling M, Van Hul E, Wuyts W, Lacza C, Dioszegi M, Dikkers FG, Hildering P, Willems PJ, Verheij JB, Lindpaintner K, Vickery B, Foernzler D, Van Hul W. Identification of a 52 kb deletion downstream of the SOST gene in patients with van Buchem disease. J Med Genet. 2002;39:91–97. - PMC - PubMed
-
- Brunkow ME, Gardner JC, Van Ness J, Paeper BW, Kovacevich BR, Proll S, Skonier JE, Zhao L, Sabo PJ, Fu Y, Alisch RS, Gillett L, Colbert T, Tacconi P, Galas D, Hamersma H, Beighton P, Mulligan J. Bone dysplasia sclerosteosis results from loss of the SOST gene product, a novel cystine knotcontaining protein. Am J Hum Genet. 2001;68:577–589. - PMC - PubMed
"VSports注册入口" Publication types
MeSH terms (V体育官网入口)
- VSports注册入口 - Actions
- "V体育安卓版" Actions
- Actions (V体育安卓版)
- Actions (VSports在线直播)
- Actions (V体育官网)
- VSports - Actions
- Actions (VSports)
- "VSports app下载" Actions
- V体育ios版 - Actions
- VSports在线直播 - Actions
- Actions (V体育官网)
- Actions (V体育平台登录)
- V体育官网入口 - Actions
- Actions (V体育官网)
- VSports最新版本 - Actions
- "VSports注册入口" Actions
- Actions (V体育官网)
Substances
- VSports app下载 - Actions
- "VSports注册入口" Actions
- VSports最新版本 - Actions
- V体育官网 - Actions
- "VSports" Actions
- "VSports手机版" Actions
- VSports注册入口 - Actions
- "V体育安卓版" Actions
VSports注册入口 - Grants and funding
"V体育官网" LinkOut - more resources
Full Text Sources (V体育官网)
Other Literature Sources
Molecular Biology Databases

