Zebrafish Naked1 and Naked2 antagonize both canonical and non-canonical Wnt signaling
- PMID: 17689523
- PMCID: "VSports最新版本" PMC2729589
- DOI: 10.1016/j.ydbio.2007.04.018
Zebrafish Naked1 and Naked2 antagonize both canonical and non-canonical Wnt signaling
Abstract
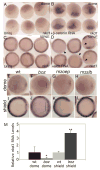
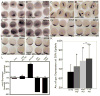
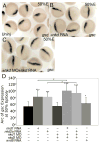
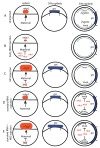
Wnt signaling controls a wide range of developmental processes and its aberrant regulation can lead to disease. To better understand the regulation of this pathway, we identified zebrafish homologues of Naked Cuticle (Nkd), Nkd1 and Nkd2, which have previously been shown to inhibit canonical Wnt/beta-catenin signaling. Zebrafish nkd1 expression increases substantially after the mid-blastula transition in a pattern mirroring that of activated canonical Wnt/beta-catenin signaling, being expressed in both the ventrolateral blastoderm margin and also in the axial mesendoderm. In contrast, zebrafish nkd2 is maternally and ubiquitously expressed VSports手机版. Overexpression of Nkd1 or Nkd2a suppressed canonical Wnt/beta-catenin signaling at multiple stages of early zebrafish development and also exacerbated the cyclopia and axial mesendoderm convergence and extension (C&E) defect in the non-canonical Wnt/PCP mutant silberblick (slb/wnt11). Thus, Nkds are sufficient to antagonize both canonical and non-canonical Wnt signaling. Reducing Nkd function using antisense morpholino oligonucleotides resulted in increased expression of canonical Wnt/beta-catenin target genes. Finally, reducing Nkd1 function in slb mutants suppressed the axial mesendoderm C&E defect. These data indicate that zebrafish Nkd1 and Nkd2 function to limit both canonical and non-canonical Wnt signaling. .
Figures



"VSports注册入口" References
-
- Angers S, Thorpe CJ, Biechele TL, Goldenberg SJ, Zheng N, MacCoss MJ, Moon RT. The KLHL12-Cullin-3 ubiquitin ligase negatively regulates the Wnt-beta-catenin pathway by targeting Dishevelled for degradation. Nat Cell Biol. 2006;8:348–357. - PubMed
-
- Bellipanni G, Varga M, Maegawa S, Imai Y, Kelly C, Myers AP, Chu F, Talbot WS, Weinberg ES. Essential and opposing roles of zebrafish beta-catenins in the formation of dorsal axial structures and neurectoderm. Development. 2006;133:1299–1309. - "VSports app下载" PubMed
-
- Cadigan KM, Nusse R. Wnt signaling: a common theme in animal development. Genes Dev. 1997;11:3286–3305. - PubMed (VSports)
Publication types
- Actions (V体育官网)
MeSH terms
- Actions (VSports注册入口)
- "VSports在线直播" Actions
- "V体育安卓版" Actions
- V体育平台登录 - Actions
- Actions (V体育2025版)
- Actions (VSports在线直播)
- Actions (VSports)
- "V体育安卓版" Actions
VSports在线直播 - Substances
- VSports app下载 - Actions
- "V体育2025版" Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

