Molecular cloning of hMena (ENAH) and its splice variant hMena+11a: epidermal growth factor increases their expression and stimulates hMena+11a phosphorylation in breast cancer cell lines (VSports)
- PMID: 17363586
- PMCID: V体育2025版 - PMC3156572
- DOI: 10.1158/0008-5472.CAN-06-1997
Molecular cloning of hMena (ENAH) and its splice variant hMena+11a: epidermal growth factor increases their expression and stimulates hMena+11a phosphorylation in breast cancer cell lines (V体育ios版)
Abstract
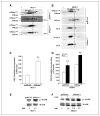
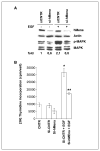
hMena (ENAH), an actin regulatory protein involved in the control of cell motility and adhesion, is modulated during human breast carcinogenesis. In fact, whereas undetectable in normal mammary epithelium, hMena becomes overexpressed in high-risk benign lesions and primary and metastatic tumors. In vivo, hMena overexpression correlates with the HER-2(+)/ER(-)/Ki67(+) unfavorable prognostic phenotype. In vitro, neuregulin-1 up-regulates whereas Herceptin treatment down-modulates hMena expression, suggesting that it may couple tyrosine kinase receptor signaling to the actin cytoskeleton. Herein, we report the cloning of hMena and of a splice variant, hMena(+11a), which contains an additional exon corresponding to 21 amino acids located in the EVH2 domain, from a breast carcinoma cell line of epithelial phenotype. Whereas hMena overexpression consistently characterizes the transformed phenotype of tumor cells of different lineages, hMena(+11a) isoform is concomitantly present only in epithelial tumor cell lines. In breast cancer cell lines, epidermal growth factor (EGF) treatment promotes concomitant up-regulation of hMena and hMena(+11a), resulting in an increase of the fraction of phosphorylated hMena(+11a) isoform only. hMena(+11a) overexpression and phosphorylation leads to increased p42/44 mitogen-activated protein kinase (MAPK) activation and cell proliferation as evidenced in hMena(+11a)-transfected breast cancer cell lines. On the contrary, hMena knockdown induces reduction of p42/44 MAPK phosphorylation and of the proliferative response to EGF. The present data provide new insight into the relevance of actin cytoskeleton regulatory proteins and, in particular, of hMena isoforms in coupling multiple signaling pathways involved in breast cancer. VSports手机版.
Figures (VSports注册入口)




References
-
- Wulfkuhle JD, Sgroi DC, Krutzsch H, et al. Proteomics of human breast ductal carcinoma in situ. Cancer Res. 2002;62:6740–9. - V体育ios版 - PubMed
-
- Wang W, Goswami S, Lapidus K, et al. Identification and testing of a gene expression signature of invasive carcinoma cells within primary mammary tumors. Cancer Res. 2004;64:8585–94. - VSports手机版 - PubMed
-
- Di Modugno F, Bronzi G, Scanlan MJ, et al. Human Mena protein, a serex-defined antigen overexpressed in breast cancer eliciting both humoral and CD8+ T-cell immune response. Int J Cancer. 2004;109:909–18. - "VSports最新版本" PubMed
-
- Di Modugno F, Mottolese M, Di Benedetto A, et al. The cytoskeleton regulatory protein hmena (ENAH) is over-expressed in human benign breast lesions with high risk of transformation and human epidermal growth factor receptor-2-positive/hormonal receptor-negative tumors. Clin Cancer Res. 2006;12:1470–8. - "VSports最新版本" PubMed
-
- Bear JE, Svitkina TM, Krause M, et al. Antagonism between Ena/VASP proteins and actin filament capping regulates fibroblast motility. Cell. 2002;109:509–21. - PubMed
Publication types
- Actions (VSports)
- Actions (VSports app下载)
MeSH terms
- VSports - Actions
- VSports最新版本 - Actions
- VSports注册入口 - Actions
- VSports手机版 - Actions
- "V体育安卓版" Actions
- Actions (VSports最新版本)
- "VSports" Actions
Substances
- Actions (V体育平台登录)
- Actions (V体育平台登录)
- Actions (VSports注册入口)
- Actions (V体育ios版)
Grants and funding
LinkOut - more resources
"V体育平台登录" Full Text Sources
"VSports手机版" Other Literature Sources
Medical
Research Materials (V体育ios版)
Miscellaneous

