p53-deficient cells rely on ATM- and ATR-mediated checkpoint signaling through the p38MAPK/MK2 pathway for survival after DNA damage
- PMID: 17292828
- PMCID: PMC2742175
- DOI: V体育平台登录 - 10.1016/j.ccr.2006.11.024
p53-deficient cells rely on ATM- and ATR-mediated checkpoint signaling through the p38MAPK/MK2 pathway for survival after DNA damage
Abstract
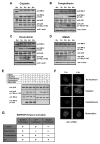
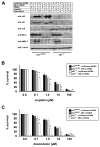
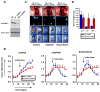
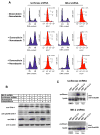
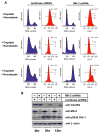
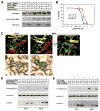
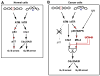
In response to DNA damage, eukaryotic cells activate ATM-Chk2 and/or ATR-Chk1 to arrest the cell cycle and initiate DNA repair. We show that, in the absence of p53, cells depend on a third cell-cycle checkpoint pathway involving p38MAPK/MK2 for cell-cycle arrest and survival after DNA damage VSports手机版. MK2 depletion in p53-deficient cells, but not in p53 wild-type cells, caused abrogation of the Cdc25A-mediated S phase checkpoint after cisplatin exposure and loss of the Cdc25B-mediated G2/M checkpoint following doxorubicin treatment, resulting in mitotic catastrophe and pronounced regression of murine tumors in vivo. We show that the Chk1 inhibitor UCN-01 also potently inhibits MK2, suggesting that its clinical efficacy results from the simultaneous disruption of two critical checkpoint pathways in p53-defective cells. .
Figures

References (V体育官网入口)
-
- Agner J, Falck J, Lukas J, Bartek J. Differential impact of diverse anticancer chemotherapeutics on the Cdc25A-degradation checkpoint pathway. Exp Cell Res. 2005;302:162–169. - PubMed
-
- Bartek J, Lukas J. Pathways governing G1/S transition and their response to DNA damage. FEBS Lett. 2001;490:117–122. - PubMed
-
- Bartek J, Lukas J. Chk1 and Chk2 kinases in checkpoint control and cancer. Cancer Cell. 2003;3:421–429. - PubMed
"VSports最新版本" Publication types
- VSports最新版本 - Actions
MeSH terms
- "V体育ios版" Actions
- V体育2025版 - Actions
- VSports app下载 - Actions
- "VSports最新版本" Actions
- Actions (V体育官网入口)
- "V体育官网入口" Actions
- Actions (V体育2025版)
- "V体育官网" Actions
- VSports注册入口 - Actions
- Actions (V体育官网入口)
- Actions (VSports在线直播)
- "VSports app下载" Actions
- Actions (VSports手机版)
- VSports最新版本 - Actions
- "V体育官网入口" Actions
- VSports app下载 - Actions
- Actions (VSports最新版本)
- VSports注册入口 - Actions
- Actions (V体育2025版)
- V体育官网 - Actions
- "VSports app下载" Actions
- "V体育官网入口" Actions
- "V体育官网入口" Actions
- V体育安卓版 - Actions
- Actions (VSports在线直播)
- "VSports" Actions
Substances
- Actions (VSports注册入口)
- V体育ios版 - Actions
- Actions (VSports手机版)
- Actions (VSports在线直播)
- "VSports注册入口" Actions
- V体育官网 - Actions
- V体育官网 - Actions
- Actions (V体育ios版)
- "V体育安卓版" Actions
Grants and funding
"VSports手机版" LinkOut - more resources
"V体育ios版" Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials (VSports手机版)
Miscellaneous

