VSports最新版本 - ATF4 mediation of NF1 functions in osteoblast reveals a nutritional basis for congenital skeletal dysplasiae
- PMID: 17141628
- PMCID: PMC2756713
- DOI: 10.1016/j.cmet.2006.10.010
ATF4 mediation of NF1 functions in osteoblast reveals a nutritional basis for congenital skeletal dysplasiae
Abstract
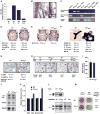
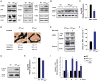
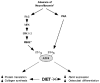
The transcription factor ATF4 enhances bone formation by favoring amino acid import and collagen synthesis in osteoblasts, a function requiring its phosphorylation by RSK2, the kinase inactivated in Coffin-Lowry Syndrome VSports手机版. Here, we show that in contrast, RSK2 activity, ATF4-dependent collagen synthesis, and bone formation are increased in mice lacking neurofibromin in osteoblasts (Nf1(ob)(-/-) mice). Independently of RSK2, ATF4 phosphorylation by PKA is enhanced in Nf1(ob)(-/-) mice, thereby increasing Rankl expression, osteoclast differentiation, and bone resorption. In agreement with ATF4 function in amino acid transport, a low-protein diet decreased bone protein synthesis and normalized bone formation and bone mass in Nf1(ob)(-/-) mice without affecting other organ weight, while a high-protein diet overcame Atf4(-/-) and Rsk2(-/-) mice developmental defects, perinatal lethality, and low bone mass. By showing that ATF4-dependent skeletal dysplasiae are treatable by dietary manipulations, this study reveals a molecular connection between nutrition and skeletal development. .
Figures



Comment in (VSports app下载)
-
Protein nutrition as therapy for a genetic disorder of bone?Cell Metab. 2006 Dec;4(6):419-20. doi: 10.1016/j.cmet.2006.11.007. Cell Metab. 2006. PMID: 17141624
References
-
- Alwan S, Tredwell SJ, Friedman JM. Is osseous dysplasia a primary feature of neurofibromatosis 1 (NF1)? Clin Genet. 2005;67:378–390. - PubMed
-
- Bashey RI, Cox R, McCann J, Jimenez SA. Changes in collagen biosynthesis, types, and mechanics of aorta in hypertensive rats. J Lab Clin Med. 1989;113:604–611. - PubMed
-
- Bialek P, Kern B, Yang X, Schrock M, Sosic D, Hong N, Wu H, Yu K, Ornitz DM, Olson EN, et al. A twist code determines the onset of osteoblast differentiation. Dev Cell. 2004;6:423–435. - PubMed
-
- Dacquin R, Starbuck M, Schinke T, Karsenty G. Mouse alpha1(I)-collagen promoter is the best known promoter to drive efficient Cre recombinase expression in osteoblast. Dev Dyn. 2002;224:245–251. - PubMed
Publication types
- Actions (V体育官网)
VSports - MeSH terms
- Actions (V体育官网)
- "VSports" Actions
- V体育安卓版 - Actions
- "VSports在线直播" Actions
- VSports在线直播 - Actions
- Actions (V体育官网)
- VSports注册入口 - Actions
- VSports app下载 - Actions
- V体育官网入口 - Actions
- Actions (VSports最新版本)
- VSports手机版 - Actions
- VSports最新版本 - Actions
- V体育安卓版 - Actions
- V体育官网 - Actions
- Actions (VSports注册入口)
Substances
- "V体育官网" Actions
- V体育安卓版 - Actions
- V体育2025版 - Actions
- V体育2025版 - Actions
- VSports - Actions
- Actions (V体育安卓版)
Grants and funding
LinkOut - more resources
"VSports注册入口" Full Text Sources
"VSports注册入口" Other Literature Sources
Medical (VSports在线直播)
"V体育安卓版" Molecular Biology Databases
Research Materials
Miscellaneous

