Cystine/glutamate exchange modulates glutathione supply for neuroprotection from oxidative stress and cell proliferation
- PMID: 17035536
- PMCID: "V体育安卓版" PMC6674710
- DOI: 10.1523/JNEUROSCI.3178-06.2006
Cystine/glutamate exchange modulates glutathione supply for neuroprotection from oxidative stress and cell proliferation (VSports app下载)
Abstract (VSports最新版本)
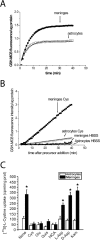
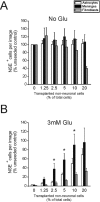
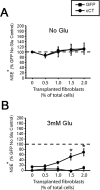
The cystine/glutamate exchanger (xCT) provides intracellular cyst(e)ine for production of glutathione, a major cellular antioxidant. Using xCT overexpression and underexpression, we present evidence that xCT-dependent glutathione production modulates both neuroprotection from oxidative stress and cell proliferation. In embryonic and adult rat brain, xCT protein was enriched at the CSF-brain barrier (i. e. , meninges) and also expressed in the cortex, hippocampus, striatum, and cerebellum. To examine the neuroprotective role of xCT, various non-neuronal cell types (astrocytes, meningeal cells, and peripheral fibroblasts) were cocultured with immature cortical neurons and exposed to oxidative glutamate toxicity, a model involving glutathione depletion. Cultured meningeal cells, which naturally maintain high xCT expression, were more neuroprotective than astrocytes. Selective xCT overexpression in astrocytes was sufficient to enhance glutathione synthesis/release and confer potent glutathione-dependent neuroprotection from oxidative stress. Moreover, normally nonprotective fibroblasts could be re-engineered to be neuroprotective with ectopic xCT overexpression indicating that xCT is a key step in the pathway to glutathione synthesis. Conversely, astrocytes and meningeal cells derived from sut/sut mice (xCT loss-of-function mutants) showed greatly reduced proliferation in culture attributable to increased oxidative stress and thiol deficiency, because growth could be rescued by the thiol-donor beta-mercaptoethanol. Strikingly, sut/sut mice developed brain atrophy by early adulthood, exhibiting ventricular enlargement, thinning of the cortex, and shrinkage of the striatum. Our results indicate that xCT can provide neuroprotection by enhancing glutathione export from non-neuronal cells such as astrocytes and meningeal cells. Furthermore, xCT is critical for cell proliferation during development in vitro and possibly in vivo VSports手机版. .
Figures




References
-
- Allen JW, Shanker G, Tan KH, Aschner M. The consequences of methylmercury exposure on interactive functions between astrocytes and neurons. Neurotoxicology. 2002;23:755–759. - PubMed (V体育2025版)
-
- Aoyama K, Suh SW, Hamby AM, Liu J, Chan WY, Chen Y, Swanson RA. Neuronal glutathione deficiency and age-dependent neurodegeneration in the EAAC1 deficient mouse. Nat Neurosci. 2006;9:119–126. - PubMed
-
- Back SA, Gan X, Li Y, Rosenberg PA, Volpe JJ. Maturation-dependent vulnerability of oligodendrocytes to oxidative stress-induced death caused by glutathione depletion. J Neurosci. 1998;18:6241–6253. - VSports最新版本 - PMC - PubMed
-
- Barger SW, Basile AS. Activation of microglia by secreted amyloid precursor protein evokes release of glutamate by cystine exchange and attenuates synaptic function. J Neurochem. 2001;76:846–854. - PubMed
-
- Bender AS, Reichelt W, Norenberg MD. Characterization of cystine uptake in cultured astrocytes. Neurochem Int. 2000;37:269–276. - PubMed
Publication types (V体育ios版)
- Actions (VSports最新版本)
MeSH terms (VSports最新版本)
- "V体育ios版" Actions
- "V体育安卓版" Actions
- "VSports手机版" Actions
- Actions (VSports)
- "VSports app下载" Actions
- "VSports" Actions
- V体育ios版 - Actions
- Actions (VSports手机版)
- "VSports" Actions
- Actions (VSports手机版)
- Actions (VSports注册入口)
Substances
- VSports注册入口 - Actions
- "V体育官网入口" Actions
V体育安卓版 - LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
