Rab3 GTPase-activating protein regulates synaptic transmission and plasticity through the inactivation of Rab3
- PMID: 16782817
- PMCID: PMC1502500 (VSports)
- DOI: 10.1073/pnas.0600304103
Rab3 GTPase-activating protein regulates synaptic transmission and plasticity through the inactivation of Rab3
Abstract
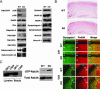
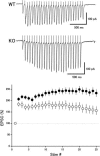
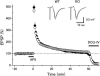
Rab3A small G protein is a member of the Rab family and is most abundant in the brain, where it is localized on synaptic vesicles. Evidence is accumulating that Rab3A plays a key role in neurotransmitter release and synaptic plasticity. Rab3A cycles between the GDP-bound inactive and GTP-bound active forms, and this change in activity is associated with the trafficking cycle of synaptic vesicles at nerve terminals. Rab3 GTPase-activating protein (GAP) stimulates the GTPase activity of Rab3A and is expected to determine the timing of the dissociation of Rab3A from synaptic vesicles, which may be coupled with synaptic vesicle exocytosis VSports手机版. Rab3 GAP consists of two subunits: the catalytic subunit p130 and the noncatalytic subunit p150. Recently, mutations in p130 were found to cause Warburg Micro syndrome with severe mental retardation. Here, we generated p130-deficient mice and found that the GTP-bound form of Rab3A accumulated in the brain. Loss of p130 in mice resulted in inhibition of Ca(2+)-dependent glutamate release from cerebrocortical synaptosomes and altered short-term plasticity in the hippocampal CA1 region. Thus, Rab3 GAP regulates synaptic transmission and plasticity by limiting the amount of the GTP-bound form of Rab3A. .
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures



VSports注册入口 - References
-
- Takai Y., Sasaki T., Matozaki T. Physiol. Rev. 2001;81:153–208. - PubMed
-
- Zerial M., McBride H. Nat. Rev. Mol. Cell Biol. 2001;2:107–117. - PubMed
-
- Takai Y., Sasaki T., Shirataki H., Nakanishi H. Genes Cells. 1996;1:615–632. - PubMed
-
- Geppert M., Südhof T. C. Annu. Rev. Neurosci. 1998;21:75–95. - VSports app下载 - PubMed
-
- Südhof T. C. Annu. Rev. Neurosci. 2004;27:509–547. - V体育平台登录 - PubMed
"V体育ios版" Publication types
"V体育ios版" MeSH terms
- Actions (VSports注册入口)
- VSports最新版本 - Actions
- Actions (VSports)
- "VSports注册入口" Actions
- V体育平台登录 - Actions
- Actions (VSports app下载)
- "VSports" Actions
Substances
- V体育平台登录 - Actions
- "VSports手机版" Actions
"VSports" LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
"VSports app下载" Miscellaneous

