Amnionless function is required for cubilin brush-border expression and intrinsic factor-cobalamin (vitamin B12) absorption in vivo
- PMID: 15845892
- PMCID: PMC1895201
- DOI: 10.1182/blood-2005-03-1197
Amnionless function is required for cubilin brush-border expression and intrinsic factor-cobalamin (vitamin B12) absorption in vivo
Abstract
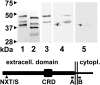
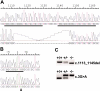
Amnionless (AMN) and cubilin gene products appear to be essential functional subunits of an endocytic receptor called cubam. Mutation of either gene causes autosomal recessive Imerslund-Gräsbeck syndrome (I-GS, OMIM no. 261100) in humans, a disorder characterized by selective intestinal malabsorption of cobalamin (vitamin B12) and urinary loss of several specific low-molecular-weight proteins. Vital insight into the molecular pathology of I-GS has been obtained from studies of dogs with a similar syndrome. In this work, we show that I-GS segregates in a large canine kindred due to an in-frame deletion of 33 nucleotides in exon 10 of AMN. In a second, unrelated I-GS kindred, affected dogs exhibit a homozygous substitution in the AMN translation initiation codon VSports手机版. Studies in vivo demonstrated that both mutations abrogate AMN expression and block cubilin processing and targeting to the apical membrane. The essential features of AMN dysfunction observed in vivo are recapitulated in a heterologous cell-transfection system, thus validating the system for analysis of AMN-cubilin interactions. Characterization of canine AMN mutations that cause I-GS establishes the canine model as an ortholog of the human disorder well suited to studies of AMN function and coevolution with cubilin. .
"V体育2025版" Figures



V体育安卓版 - References
-
- Rosenblatt DS, Fenton WA. Inborn errors of cobalamin metabolism. In: Banerjee R, ed. Chemistry and Biochemistry of Vitamin B12. New York, NY: John Wiley and Sons; 1999: 367-384.
-
- Imerslund O. Idiopathic chronic megaloblastic anemia in children. Acta Paediatr Scand. 1960;49(suppl 119): 1-115. - PubMed
-
- Gräsbeck R, Gordin R, Kantero I, Kuhlback B. Selective vitamin B12 malabsorption and proteinuria in young people. Acta Med Scand. 1960;167: 289-296. - PubMed
-
- Aminoff M, Carter JE, Chadwick RB, et al. Mutations in CUBN, encoding the intrinsic factor-vitamin B12 receptor, cubilin, cause hereditary megaloblastic anaemia 1. Nat Genet. 1999;21: 309-313. - PubMed
-
- Tanner SM, Aminoff M, Wright FA, et al. Amnionless, essential for mouse gastrulation, is mutated in recessive hereditary megaloblastic anemia. Nat Genet. 2003;33: 426-429. - PubMed
Publication types
- "V体育ios版" Actions
- Actions (VSports手机版)
MeSH terms
- "V体育平台登录" Actions
- VSports在线直播 - Actions
- "V体育2025版" Actions
- "V体育官网入口" Actions
- Actions (V体育ios版)
- VSports最新版本 - Actions
- "VSports最新版本" Actions
- V体育官网入口 - Actions
- VSports - Actions
Substances
- Actions (V体育官网入口)
- VSports最新版本 - Actions
- VSports在线直播 - Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

