A systemic small RNA signaling system in plants
- PMID: 15258266
- PMCID: PMC519190
- DOI: 10.1105/tpc.104.023614
A systemic small RNA signaling system in plants
Abstract
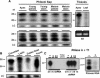
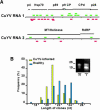
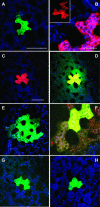
Systemic translocation of RNA exerts non-cell-autonomous control over plant development and defense. Long-distance delivery of mRNA has been proven, but transport of small interfering RNA and microRNA remains to be demonstrated. Analyses performed on phloem sap collected from a range of plants identified populations of small RNA species VSports手机版. The dynamic nature of this population was reflected in its response to growth conditions and viral infection. The authenticity of these phloem small RNA molecules was confirmed by bioinformatic analysis; potential targets for a set of phloem small RNA species were identified. Heterografting studies, using spontaneously silencing coat protein (CP) plant lines, also established that transgene-derived siRNA move in the long-distance phloem and initiate CP gene silencing in the scion. Biochemical analysis of pumpkin (Cucurbita maxima) phloem sap led to the characterization of C. maxima Phloem SMALL RNA BINDING PROTEIN1 (CmPSRP1), a unique component of the protein machinery probably involved in small RNA trafficking. Equivalently sized small RNA binding proteins were detected in phloem sap from cucumber (Cucumis sativus) and lupin (Lupinus albus). PSRP1 binds selectively to 25-nucleotide single-stranded RNA species. Microinjection studies provided direct evidence that PSRP1 could mediate the cell-to-cell trafficking of 25-nucleotide single-stranded, but not double-stranded, RNA molecules. The potential role played by PSRP1 in long-distance transmission of silencing signals is discussed with respect to the pathways and mechanisms used by plants to exert systemic control over developmental and physiological processes. .
Figures








References (V体育官网入口)
-
- Atkins, C.A. (1999). Spontaneous phloem exudation accompanying abscission in Lupinus mutabilis (Sweet). J. Exp. Bot. 50, 805–812.
-
- Aukerman, M.J., and Sakai, H. (2003). Regulation of flowering time and floral organ identity by a microRNA and its APETALA2-like target genes. Plant Cell 15, 2730–2741. - V体育平台登录 - PMC - PubMed
-
- Bartel, B., and Bartel, D.P. (2003). MicroRNAs: At the root of plant development? Plant Physiol. 132, 709–717. - V体育官网 - PMC - PubMed
Publication types (V体育安卓版)
"V体育2025版" MeSH terms
- Actions (VSports app下载)
- "VSports" Actions
- "VSports app下载" Actions
- "V体育2025版" Actions
- V体育平台登录 - Actions
- VSports在线直播 - Actions
- Actions (VSports注册入口)
- V体育安卓版 - Actions
- "V体育ios版" Actions
- "VSports" Actions
- "VSports注册入口" Actions
Substances
- VSports - Actions
- "VSports" Actions
Associated data
- Actions (VSports手机版)
- Actions
- "V体育官网入口" Actions
- V体育平台登录 - Actions
- "V体育官网入口" Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources (VSports手机版)
Molecular Biology Databases
Miscellaneous (V体育安卓版)

