"VSports手机版" Alternative splicing of N- and C-termini of a C. elegans ClC channel alters gating and sensitivity to external Cl- and H+
- PMID: 14565992
- PMCID: PMC1664825
- DOI: 10.1113/jphysiol.2003.053165
Alternative splicing of N- and C-termini of a C. elegans ClC channel alters gating and sensitivity to external Cl- and H+
Abstract
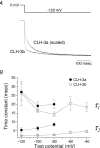
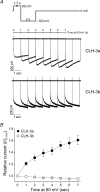
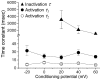
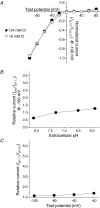
CLH-3 is a meiotic cell cycle-regulated ClC Cl- channel that is functionally expressed in oocytes of the nematode Caenorhabditis elegans. CLH-3a and CLH-3b are alternatively spliced variants that have identical intramembrane regions, but which exhibit striking differences in their N- and C-termini. Structural and functional studies indicate that N- and C-terminal domains modulate ClC channel activity. We therefore postulated that alternative splicing of CLH-3 would alter channel gating and physiological functions. To begin testing this hypothesis, we characterized the biophysical properties of CLH-3a and CLH-3b expressed heterologously in HEK293 cells. CLH-3a activates more slowly and requires stronger hyperpolarization for activation than CLH-3b. Depolarizing conditioning voltages dramatically increase CLH-3a current amplitude and induce a slow inactivation process at hyperpolarized voltages, but have no significant effect on CLH-3b activity VSports手机版. CLH-3a also differs significantly in its extracellular Cl- and pH sensitivity compared to CLH-3b. Immunofluorescence microscopy demonstrated that CLH-3b is translationally expressed during all stages of oocyte development, and furthermore, the biophysical properties of the native oocyte Cl- current are indistinguishable from those of heterologously expressed CLH-3b. We conclude that CLH-3b carries the oocyte Cl- current and that the channel probably functions in nonexcitable cells to depolarize membrane potential and/or mediate net Cl- transport. The unique voltage-dependent properties of CLH-3a suggest that the channel may function in muscle cells and neurones to regulate membrane excitability. We suggest that alternative splicing of CLH-3 N- and C-termini modifies the functional properties of the channel by altering the accessibility and/or function of pore-associated ion-binding sites. .
Figures








References
-
- Adrian RH, Bryant SH. On the repetitive discharge in myotonic muscle fibres. J Physiol. 1974;240:505–515. - V体育官网入口 - PMC - PubMed
-
- Bargmann CI. Neurobiology of the Caenorhabditis elegans genome. Science. 1998;282:2028–2033. - PubMed
-
- Chen TY, Miller C. gating and voltage dependence of the ClC-0 Cl− channel. J General Physiol. 1996;108:237–250. - PMC (V体育安卓版) - PubMed
Publication types
MeSH terms (V体育官网入口)
- V体育平台登录 - Actions
- V体育官网入口 - Actions
- Actions (VSports手机版)
- VSports最新版本 - Actions
- "V体育2025版" Actions
- V体育ios版 - Actions
- "V体育2025版" Actions
- "V体育平台登录" Actions
- Actions (V体育官网入口)
- "VSports在线直播" Actions
Substances
- V体育2025版 - Actions
- Actions (VSports)
- Actions (V体育官网)
Grants and funding
V体育平台登录 - LinkOut - more resources
Full Text Sources
Molecular Biology Databases
