Dwarfism, impaired skin development, skeletal muscle atrophy, delayed bone development, and impeded adipogenesis in mice lacking Akt1 and Akt2
- PMID: 12782654
- PMCID: PMC196068
- DOI: "V体育安卓版" 10.1101/gad.1089403
Dwarfism, impaired skin development, skeletal muscle atrophy, delayed bone development, and impeded adipogenesis in mice lacking Akt1 and Akt2
Abstract
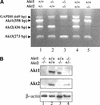
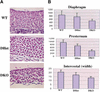
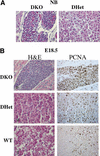
To elucidate the functions of the serine/threonine kinase Akt/PKB in vivo, we generated mice lacking both akt1 and akt2 genes. Akt1/Akt2 double-knockout (DKO) mice exhibit severe growth deficiency and die shortly after birth. These mice display impaired skin development because of a proliferation defect, severe skeletal muscle atrophy because of a marked decrease in individual muscle cell size, and impaired bone development. These defects are strikingly similar to the phenotypes of IGF-1 receptor-deficient mice and suggest that Akt may serve as the most critical downstream effector of the IGF-1 receptor during development. In addition, Akt1/Akt2 DKO mice display impeded adipogenesis VSports手机版. Specifically, Akt1 and Akt2 are required for the induced expression of PPARgamma, the master regulator of adipogenesis, establishing a new essential role for Akt in adipocyte differentiation. Overall, the combined deletion of Akt1 and Akt2 establishes in vivo roles for Akt in cell proliferation, growth, and differentiation. These functions of Akt were uncovered despite the observed lower level of Akt activity mediated by Akt3 in Akt1/Akt2 DKO cells, suggesting that a critical threshold level of Akt activity is required to maintain normal cell proliferation, growth, and differentiation. .
Figures







References
-
- Altomare D.A., Guo, K., Cheng, J.Q., Sonoda, G., Walsh, K., and Testa, J.R. 1995. Cloning, chromosomal localization and expression analysis of the mouse Akt2 oncogene. Oncogene 11: 1055–1060. - PubMed
-
- Altomare D.A., Lyons, G.E., Mitsuuchi, Y., Cheng, J.Q., and Testa, J.R. 1998. Akt2 mRNA is highly expressed in embryonic brown fat and the AKT2 kinase is activated by insulin. Oncogene 116: 2407–2411. - PubMed
-
- Barak Y., Nelson, M.C., Ong, E.S., Jones, Y.Z., Ruiz-Lozano, P., Chien, K.R., Koder, A., and Evans, R.M. 1999. PPAR γ is required for placental, cardiac, and adipose tissue development. Mol. Cell 4: 585–595. - V体育ios版 - PubMed
-
- Bodine S.C., Stitt, T.N., Gonzalez, M., Kline, W.O., Stover, G.L., Bauerlein, R., Zlotchenko, E., Scrimgeour, A., Lawrence, J.C., Glass, D.J., et al. 2001. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat. Cell Biol. 3: 1014–1019. - PubMed
-
- Brazil D.P. and Hemmings, B.A. 2001. Ten years of protein kinase B signalling: Ahard Akt to follow. Trends Biochem. Sci. 26: 657–664. - PubMed
Publication types
- VSports注册入口 - Actions
MeSH terms
- Actions (VSports手机版)
- Actions (V体育ios版)
- V体育平台登录 - Actions
- "VSports手机版" Actions
- Actions (VSports app下载)
- "VSports手机版" Actions
- "VSports注册入口" Actions
- "V体育官网" Actions
- Actions (V体育安卓版)
- Actions (V体育平台登录)
- Actions (V体育平台登录)
- VSports在线直播 - Actions
- Actions (V体育平台登录)
- "V体育安卓版" Actions
- "VSports手机版" Actions
Substances
- Actions (VSports最新版本)
- Actions (V体育官网)
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases (VSports在线直播)
Miscellaneous
