Human OGG1 undergoes serine phosphorylation and associates with the nuclear matrix and mitotic chromatin in vivo
- PMID: 12034821
- PMCID: PMC117190
- DOI: 10.1093/nar/30.11.2349
Human OGG1 undergoes serine phosphorylation and associates with the nuclear matrix and mitotic chromatin in vivo (VSports在线直播)
Abstract
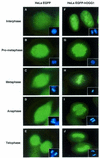
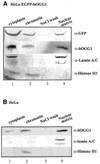
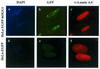
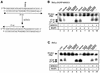
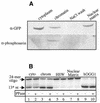
OGG1 is the major DNA glycosylase in human cells for removal of 7,8 dihydro-8-oxoguanine (8-oxoG), one of the most frequent endogenous base lesions formed in the DNA of aerobic organisms. During replication, 8-oxoG will frequently mispair with adenine, thus forming G:C --> T:A transversions, a common somatic mutation associated with human cancers. In the present study, we have constructed a stable transfectant cell line expressing hOGG1 fused at the C-terminal end to green fluorescent protein (GFP) and investigated the cellular distribution of the fusion protein by fluorescence analysis. It is shown that hOGG1 is preferentially associated with chromatin and the nuclear matrix during interphase and becomes associated with the condensed chromatin during mitosis. Chromatin-bound hOGG1 was found to be phosphorylated on a serine residue in vivo as revealed by staining with an anti-phosphoserine-specific antibody. Chromatin-associated hOGG1 was co-precipitated with an antibody against protein kinase C (PKC), suggesting that PKC is responsible for the phosphorylation event. Both purified and nuclear matrix-associated hOGG1 were shown to be substrates for PKC-mediated phosphorylation in vitro. This appears to be the first demonstration of a post-translational modification of hOGG1 in vivo VSports手机版. .
Figures


References
-
- Marnett L.J. (2000) Oxyradicals and DNA damage. Carcinogenesis, 21, 361–370. - PubMed
-
- Raha S. and Robinson,B.H. (2000) Mitochondria, oxygen free radicals diseases and ageing. Trends Biochem. Sci., 25, 502–508. - PubMed (V体育ios版)
-
- Seeberg E., Eide,L. and Bjørås,M. (1995) The base excision repair pathway. Trends Biochem. Sci., 20, 391–397. - PubMed
-
- Cheng K.C., Cahill,D.S., Kasai,H., Nishimura,S. and Loeb,L.A. (1992) 8-Hydroxyguanine, an abundant form of oxidative DNA damage, causes G-T and A-C substitutions. J. Biol. Chem. 267, 166–172. - PubMed
-
- Moriya M. (1993) Single-stranded shuttle phagemid for mutagenesis studies in mammalian cells: 8-oxoguanine in DNA induces targeted G.C → T.A transversions in simian kidney cells. Proc. Natl Acad. Sci. USA, 90, 1122–1126. - "V体育平台登录" PMC - PubMed
Publication types
- "VSports在线直播" Actions
MeSH terms (VSports最新版本)
- Actions (VSports注册入口)
- VSports在线直播 - Actions
- VSports注册入口 - Actions
- "VSports注册入口" Actions
- Actions (V体育平台登录)
- VSports注册入口 - Actions
- Actions (V体育2025版)
- V体育官网 - Actions
- "VSports在线直播" Actions
Substances
- "VSports在线直播" Actions
- "VSports最新版本" Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
V体育官网 - Research Materials

