A conserved docking site in MEKs mediates high-affinity binding to MAP kinases and cooperates with a scaffold protein to enhance signal transmission
- PMID: 11134045
- PMCID: PMC3021106
- DOI: 10.1074/jbc.M010271200
"VSports手机版" A conserved docking site in MEKs mediates high-affinity binding to MAP kinases and cooperates with a scaffold protein to enhance signal transmission
Abstract
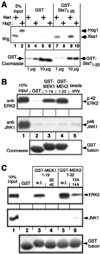
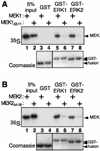
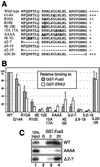
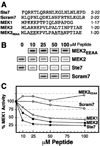
The recognition of mitogen-activated protein kinases (MAPKs) by their upstream activators, MAPK/ERK kinases (MEKs), is crucial for the effective and accurate transmission of many signals. We demonstrated previously that the yeast MAPKs Kss1 and Fus3 bind with high affinity to the N terminus of the MEK Ste7, and proposed that a conserved motif in Ste7, the MAPK-docking site, mediates this interaction. Here we show that the corresponding sequences in human MEK1 and MEK2 are necessary and sufficient for the direct binding of the MAPKs ERK1 and ERK2. Mutations in MEK1, MEK2, or Ste7 that altered conserved residues in the docking site diminished binding of the cognate MAPKs. Furthermore, short peptides corresponding to the docking sites in these MEKs inhibited MEK1-mediated phosphorylation of ERK2 in vitro. In yeast cells, docking-defective alleles of Ste7 were modestly compromised in their ability to transmit the mating pheromone signal. This deficiency was dramatically enhanced when the ability of the Ste5 scaffold protein to associate with components of the MAPK cascade was also compromised. Thus, both the MEK-MAPK docking interaction and binding to the Ste5 scaffold make mutually reinforcing contributions to the efficiency of signaling by this MAPK cascade in vivo VSports手机版. .
Figures

References
-
- Graves JD, Krebs EG. Pharmacol. Ther. 1999;82:111–121. - PubMed
-
- Hunter T. Cell. 2000;100:113–127. - PubMed
-
- Lewis TS, Shapiro PS, Ahn NG. Adv. Cancer Res. 1998;74:49–139. - VSports手机版 - PubMed
-
- Garrington T, Johnson G. Curr. Opin. Cell Biol. 1999;11:211–218. - "VSports注册入口" PubMed
Publication types
MeSH terms
- VSports手机版 - Actions
- VSports app下载 - Actions
- Actions (VSports最新版本)
- VSports最新版本 - Actions
- "V体育官网" Actions
- "V体育官网入口" Actions
- Actions (VSports手机版)
- Actions (V体育ios版)
- "VSports在线直播" Actions
- Actions (V体育2025版)
- V体育官网入口 - Actions
- V体育平台登录 - Actions
- "V体育官网" Actions
- VSports最新版本 - Actions
- V体育官网入口 - Actions
- Actions (V体育2025版)
VSports - Substances
- "VSports" Actions
- VSports手机版 - Actions
- "V体育官网入口" Actions
- Actions (V体育ios版)
- "V体育ios版" Actions
Grants and funding
LinkOut - more resources
"V体育官网" Full Text Sources
Other Literature Sources
Molecular Biology Databases (V体育官网入口)
Miscellaneous

