Smad4/DPC4-mediated tumor suppression through suppression of angiogenesis
- PMID: 10944227
- PMCID: PMC16915
- DOI: "V体育平台登录" 10.1073/pnas.97.17.9624
"VSports" Smad4/DPC4-mediated tumor suppression through suppression of angiogenesis
Abstract
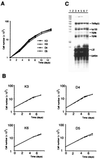
Smad4/DPC4 (deleted in pancreatic carcinoma, locus 4) is a tumor suppressor gene lost at high frequency in cancers of the pancreas and other gastrointestinal organs. Smad4 encodes a key intracellular messenger in the transforming growth factor beta (TGF-beta) signaling cascade. TGF-beta is a potent inhibitor of the growth of epithelial cells; thus, it has been assumed that loss of Smad4 during tumor progression relieves this inhibition. Herein, we show that restoration of Smad4 to human pancreatic carcinoma cells suppressed tumor formation in vivo, yet it did not restore sensitivity to TGF-beta. Rather, Smad4 restoration influenced angiogenesis, decreasing expression of vascular endothelial growth factor and increasing expression of thrombospondin-1. In contrast to the parental cell line and to control transfectants that produced rapidly growing tumors in vivo, Smad4 revertants induced small nonprogressive tumors with reduced vascular density. These data define the control of an angiogenic switch as an alternative, previously unknown mechanism of tumor suppression for Smad4 and identify the angiogenic mediators vascular endothelial growth factor and thrombospondin-1 as key target genes VSports手机版. .
Figures

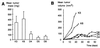
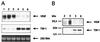



References
-
- Hahn S A, Schutte M, Hoque A T M, Moskaluk C A, da Costa L T, Rozenblum E, Weinstein C L, Fischer A, Yeo C J, Hruban R H, et al. Science. 1996;271:350–353. - V体育平台登录 - PubMed
-
- Schutte M, Hruban R H, Hedrick L, Cho K R, Nadasdy G M, Weinstein C L, Bova G S, Isaacs W B, Cairns P, Nawroz H, et al. Cancer Res. 1996;56:2527–2530. - VSports最新版本 - PubMed
-
- Bartsch D, Hahn S A, Danichevski K D, Ramaswamy A, Bastian D, Galehdari H, Barth P, Schmiegel W, Simon B, Rothmund S. Oncogene. 1999;18:2367–2371. - PubMed
-
- Hahn S A, Bartsch D, Schroers A, Galehdari H, Becker M, Ramaswamy A, Schwarte-Waldhoff I, Maschek H, Schmiegel W. Cancer Res. 1998;58:1124–1126. - PubMed
-
- Goggins M, Shekher M, Turnacioglu K, Yeo C J, Hruban R H, Kern S E. Cancer Res. 1998;58:5329–5332. - VSports手机版 - PubMed
Publication types (VSports最新版本)
MeSH terms
- Actions (VSports注册入口)
- "VSports注册入口" Actions
- VSports最新版本 - Actions
- Actions (VSports注册入口)
- VSports app下载 - Actions
- VSports手机版 - Actions
- "V体育ios版" Actions
- Actions (V体育2025版)
- Actions (VSports注册入口)
- VSports最新版本 - Actions
- V体育安卓版 - Actions
- V体育2025版 - Actions
- "VSports最新版本" Actions
- Actions (V体育ios版)
- VSports app下载 - Actions
- "VSports手机版" Actions
- "VSports手机版" Actions
- "V体育官网入口" Actions
"VSports" Substances
- V体育安卓版 - Actions
- "VSports" Actions
- Actions (VSports最新版本)
- Actions (VSports app下载)
- Actions (VSports)
- Actions (V体育平台登录)
- V体育ios版 - Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

