Acute decrease in net glutamate uptake during energy deprivation
- PMID: 10805815
- PMCID: PMC25876
- DOI: V体育安卓版 - 10.1073/pnas.97.10.5610
Acute decrease in net glutamate uptake during energy deprivation
Abstract
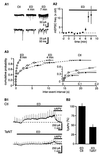
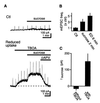
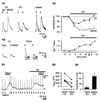
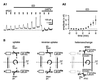
The extracellular glutamate concentration ([glu](o)) rises during cerebral ischemia, reaching levels capable of inducing delayed neuronal death. The mechanisms underlying this glutamate accumulation remain controversial. We used N-methyl-D-aspartate receptors on CA3 pyramidal neurons as a real-time, on-site, glutamate sensor to identify the source of glutamate release in an in vitro model of ischemia. Using glutamate and L-trans-pyrrolidine-2,4-dicarboxylic acid (tPDC) as substrates and DL-threo-beta-benzyloxyaspartate (TBOA) as an inhibitor of glutamate transporters, we demonstrate that energy deprivation decreases net glutamate uptake within 2-3 min and later promotes reverse glutamate transport. This process accounts for up to 50% of the glutamate accumulation during energy deprivation. Enhanced action potential-independent vesicular release also contributes to the increase in [glu](o), by approximately 50%, but only once glutamate uptake is inhibited. These results indicate that a significant rise in [glu](o) already occurs during the first minutes of energy deprivation and is the consequence of reduced uptake and increased vesicular and nonvesicular release of glutamate VSports手机版. .
Figures

"V体育官网入口" References
-
- Choi D W, Rothman S M. Annu Rev Neurosci. 1990;13:171–182. - PubMed
-
- Martin R L, Lloyd H G, Cowan A I. Trends Neurosci. 1994;17:251–257. - PubMed
-
- Obrenovitch T P, Urenjak J. Prog Neurobiol. 1997;51:39–87. - PubMed (VSports app下载)
-
- Drejer J, Benveniste H, Diemer N H, Schousboe A. J Neurochem. 1985;45:145–151. - PubMed
-
- Bosley T M, Woodhams P L, Gordon R D, Balazs R. J Neurochem. 1983;40:189–201. - PubMed
"V体育官网入口" Publication types
VSports - MeSH terms
- "VSports" Actions
- "V体育平台登录" Actions
- VSports在线直播 - Actions
- Actions (VSports)
- Actions (VSports app下载)
- "VSports最新版本" Actions
- "V体育官网入口" Actions
- "V体育官网" Actions
- "VSports在线直播" Actions
- Actions (VSports)
- Actions (V体育ios版)
- VSports手机版 - Actions
- V体育ios版 - Actions
Substances
- Actions (V体育安卓版)
- "VSports app下载" Actions
- "VSports最新版本" Actions
- "V体育安卓版" Actions
- "V体育官网" Actions
- Actions (VSports)
- VSports手机版 - Actions
- Actions (VSports最新版本)
- Actions (VSports在线直播)
"VSports手机版" LinkOut - more resources
Full Text Sources
Miscellaneous

