Molecular determinants that mediate selective activation of p38 MAP kinase isoforms
- PMID: 10716930
- PMCID: PMC305671
- DOI: 10.1093/emboj/19.6.1301 (V体育ios版)
Molecular determinants that mediate selective activation of p38 MAP kinase isoforms
Abstract
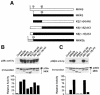
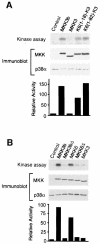
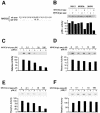
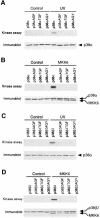
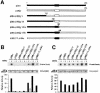
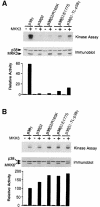
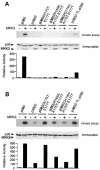
The p38 mitogen-activated protein kinase (MAPK) group is represented by four isoforms in mammals (p38alpha, p38beta2, p38gamma and p38delta). These p38 MAPK isoforms appear to mediate distinct functions in vivo due, in part, to differences in substrate phosphorylation by individual p38 MAPKs and also to selective activation by MAPK kinases (MAPKKs). Here we report the identification of two factors that contribute to the specificity of p38 MAPK activation. One mechanism of specificity is the selective formation of functional complexes between MAPKK and different p38 MAPKs. The formation of these complexes requires the presence of a MAPK docking site in the N-terminus of the MAPKK VSports手机版. The second mechanism that confers signaling specificity is the selective recognition of the activation loop (T-loop) of p38 MAPK isoforms. Together, these processes provide a mechanism that enables the selective activation of p38 MAPK in response to activated MAPKK. .
Figures


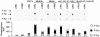

References (VSports app下载)
-
- Bardwell L. and Thorner, J. (1996) A conserved motif at the amino termini of MEKs might mediate high-affinity interaction with the cognate MAPKs. Trends Biochem. Sci., 21, 373–374. - PubMed
-
- Bardwell L., Cook, J.G., Chang, E.C., Cairns, B.R. and Thorner, J. (1996) Signaling in the yeast pheromone response pathway: specific and high-affinity interaction of the mitogen-activated protein (MAP) kinases Kss1 and Fus3 with the upstream MAP kinase kinase Ste7. Mol. Cell. Biol., 16, 3637–3650. - VSports注册入口 - PMC - PubMed
-
- Brunet A. and Pouyssegur, J. (1996) Identification of MAP kinase domains by redirecting stress signals into growth factor responses. Science, 272, 1652–1655. - PubMed
-
- Camps M., Nichols, A., Gillieron, C., Antonsson, B., Muda, M., Chabert, C., Boschert, U. and Arkinstall, S. (1998) Catalytic activation of the phosphatase MKP-3 by ERK2 mitogen-activated protein kinase. Science, 280, 1262–1265. - VSports注册入口 - PubMed
-
- Chow C.W., Rincon, M., Cavanagh, J., Dickens, M. and Davis, R.J. (1997) Nuclear accumulation of NFAT4 opposed by the JNK signal transduction pathway. Science, 278, 1638–1641. - PubMed
Publication types
- Actions (V体育官网)
MeSH terms
- Actions (V体育官网入口)
- "V体育官网入口" Actions
- "V体育平台登录" Actions
- V体育2025版 - Actions
- VSports在线直播 - Actions
- Actions (V体育官网)
- "VSports注册入口" Actions
- Actions (V体育官网入口)
- Actions (VSports)
- "VSports最新版本" Actions
- Actions (V体育安卓版)
- "VSports app下载" Actions
- "VSports最新版本" Actions
- "VSports最新版本" Actions
Substances
- VSports在线直播 - Actions
- V体育官网 - Actions
- "VSports在线直播" Actions
- VSports在线直播 - Actions
- VSports - Actions
Grants and funding
LinkOut - more resources
Full Text Sources (VSports在线直播)
Other Literature Sources
Research Materials (VSports在线直播)

