"VSports手机版" Interactions among enzymes of the Arabidopsis flavonoid biosynthetic pathway
- PMID: 10536025
- PMCID: PMC23169
- DOI: 10.1073/pnas.96.22.12929
Interactions among enzymes of the Arabidopsis flavonoid biosynthetic pathway
"VSports" Abstract
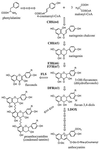
Flavonoids are secondary metabolites derived from phenylalanine and acetate metabolism that perform a variety of essential functions in higher plants. Studies over the past 30 years have supported a model in which flavonoid metabolism is catalyzed by an enzyme complex localized to the endoplasmic reticulum [Hrazdina, G. & Wagner, G. J. (1985) Arch. Biochem. Biophys. 237, 88-100]. To test this model further we assayed for direct interactions between several key flavonoid biosynthetic enzymes in developing Arabidopsis seedlings. Two-hybrid assays indicated that chalcone synthase, chalcone isomerase (CHI), and dihydroflavonol 4-reductase interact in an orientation-dependent manner. Affinity chromatography and immunoprecipitation assays further demonstrated interactions between chalcone synthase, CHI, and flavonol 3-hydroxylase in lysates from Arabidopsis seedlings. These results support the hypothesis that the flavonoid enzymes assemble as a macromolecular complex with contacts between multiple proteins. Evidence was also found for posttranslational modification of CHI VSports手机版. The importance of understanding the subcellular organization of elaborate enzyme systems is discussed in the context of metabolic engineering. .
Figures




References
-
- Zalokar M. Exp Cell Res. 1960;19:114–132. - PubMed
-
- Kempner E S, Miller J H. Exp Cell Res. 1968;51:141–149. - PubMed
-
- Ovádi J, Srere P A. Cell Biochem Funct. 1996;14:249–258. - VSports app下载 - PubMed
-
- Mathews C K. J Bacteriol. 1993;175:6377–6381. - VSports - PMC - PubMed
-
- Sui D, Wilson J E. Arch Biochem Biophys. 1997;345:111–125. - PubMed
Publication types
MeSH terms
- VSports注册入口 - Actions
- VSports - Actions
- "V体育安卓版" Actions
- Actions (V体育官网入口)
- Actions (V体育2025版)
- "VSports注册入口" Actions
- "V体育安卓版" Actions
Substances (V体育安卓版)
- Actions (VSports手机版)
- Actions (VSports app下载)
- Actions (VSports)
"V体育2025版" LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

