Resveratrol Chemosensitizes TNF-β-Induced Survival of 5-FU-Treated Colorectal Cancer Cells
"> Figure 1
Effect of resveratrol and/or 5-FU on CRC cell migration induced by TNF-β or TNF-α in inflammatory microenvironment. Serum-starved HCT116 (A) and HCT116R (B) were cultured in alginate culture and treated as described in detail in “Section 2”. Invasive colonies were stained with toluidine blue and the number of emigrated spheroids was quantified after 10 days in culture. Each experiment was repeated at least three times and experimental values were compared with the control and statistically significant values with p < 0.05 were designated by an asterisk (*) and p < 0.01 were designated by two asterisks (**).
"> Figure 2(A,B): Effect of resveratrol on CSC formation in TNF-β-induced inflammatory microenvironment. Serum-starved HCT116 and HCT116R were cultured in alginate culture and treated as described in detail in “Section 2”. Invasive cells from the alginate that adhered at the bottom of the petri dish and formed colonies were subjected to immunolabeling with primary antibodies against CD44 (A) and ALDH1 (B) followed by incubation with rhodamine-coupled secondary antibodies and counterstaining with DAPI to visualize cell nuclei. Images shown are representative of three different experiments. Magnification 600×; bar = 30 nm. The number of positively stained cells was quantified by counting 300 cells from ten different microscopic fields of view. The values were compared to the control and statistically-significant values with p < 0.05 and significant values are marked with an asterisk (*) and p < 0.01 were designated by two asterisks (**). (C,D): Effect of resveratrol and/or 5-FU on cancer stem cell formation induced by TNF-β in CRC cells in inflammatory microenvironment culture. Serum-starved HCT116 (C) and HCT116R (D) in alginate culture were treated as described in detail in “Section 2”. After 10 days whole cell lysates were prepared and western blotting performed with antibodies against ALDH1, CD44 and CD133. Western blots shown are representative of three independent experiments. The housekeeping protein β-actin served as a positive loading control in all experiments.
"> Figure 3Effect of resveratrol and/or 5-FU on apoptosis induced by TNF-β in HCT116 and HCT116R cells. Serum-starved HCT116 (I,II) and HCT116R (II) were cultured on glass plates and treated as described in detail in “Section 2” for 72 h and DAPI nuclear staining assay was performed to determine the amount of apoptotic nuclei. II: The number of the apoptotic nuclei was quantified by counting 800 cells from 20 microscopic fields. The examination was performed in triplicate and the results are provided as mean values with standard deviations p < 0.05 are designated by an asterisk (*); p < 0.01 by two asterisks (**). Magnification: 400×.
"> Figure 4Ultrastructural demonstration of cell viability and apoptosis of CRC cells after treatment with resveratrol and/or 5-FU in TNF-β-induced inflammatory microenvironment. Serum-starved HCT116 (I,II) and HCT116R (II) were cultured in alginate culture and treated as described in detail in “Section 2” for 10 days and spheroid formation and apoptotic induction investigated. Magnification: ×5000, bar = 1 μM. II: The number of apoptotic cells was quantified by counting 300 cells from 20 different microscopic fields. Values were compared to the control, and statistically-significant values were labelled with p < 0.05 are designated by an asterisk (*); p < 0.01 by two asterisks (**).
"> Figure 5(A,B): Effect of Resveratrol and/or 5-FU on NF-κB activation and NF-κB-regulated gene end-products involved in apoptosis, metastasis induced by TNF-β in HCT116 and HCT116R in inflammatory microenvironment. Alginate cultures of HCT116 (A) and HCT116R (B) were treated for 10 days as described in detail in “Section 2”. Immunoblotting with whole cell lysates was performed with antibodies against p65-NF-κB, CXCR4, MMP-9 and cleaved-caspase-3. Western blots shown are representative of three independent experiments. The housekeeping protein β-actin served as a positive loading control in all experiments. (C,D): Effect of resveratrol and/or 5-FU on TNF-β-induced epithelial-to-mesenchymal transition of CRC cells in tumor microenvironment cultures. Colorectal cancer cells in alginate culture were treated as described in Materials and Methods. After 10 days whole cell lysates of HCT116 (C) and HCT116R (D) were subjected to western blotting with antibodies against vimentin, E-cadherin and slug. Western blots shown are representative of three independent experiments. The housekeeping protein β-actin served as a positive loading control in all experiments.
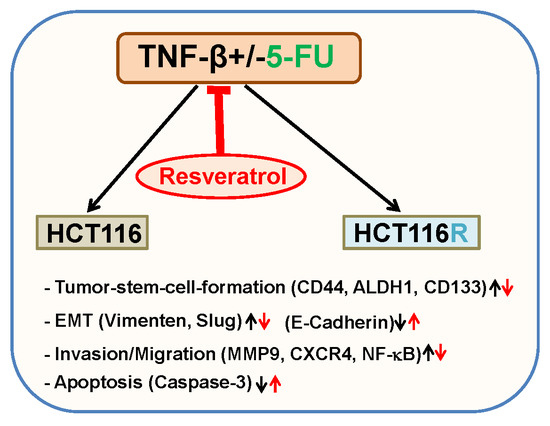
"> Figure 6Schematic diagram showing modulatory effect of resveratrol in TNF-β-mediated pro-inflammatory tumor microenvironment on malignity of 5-FU resistant and non-resistant CRC cells.
">
Abstract
Objective: Resveratrol, a safe and multitargeted natural agent, has been linked with inhibition of survival and invasion of tumor cells. Tumor Necrosis Factor-β (TNF-β) (Lymphotoxin α) is known as an inflammatory cytokine, however, the underlying mechanisms for its pro-carcinogenic effects and whether resveratrol can suppress these effects in the tumor microenvironment are poorly understood. Methods: We investigated whether resveratrol modulates the effects of 5-Fluorouracil (5-FU) and TNF-β on the malignant potential of human colorectal cancer (CRC) cells (HCT116) and their corresponding isogenic 5-FU-chemoresistant derived clones (HCT116R) in 3D-alginate tumor microenvironment. Results: CRC cells cultured in alginate were able to migrate from alginate and the numbers of migrated cells were significantly increased in the presence of TNF-β, similar to TNF-α, and dramatically decreased by resveratrol V体育官网入口. We found that TNF-β promoted chemoresistance in CRC cells to 5-FU compared to control cultures and resveratrol chemosensitizes TNF-β-induced increased capacity for survival and invasion of HCT116 and HCT116R cells to 5-FU. Furthermore, TNF-β induced a more pronounced cancer stem cell-like (CSC) phenotype (CD133, CD44, ALDH1) and resveratrol suppressed formation of CSC cells in two different CRC cells and this was accompanied with a significant increase in apoptosis (caspase-3). It is noteworthy that resveratrol strongly suppressed TNF-β-induced activation of tumor-promoting factors (NF-κB, MMP-9, CXCR4) and epithelial-to-mesenchymal-transition-factors (increased vimentin and slug, decreased E-cadherin) in CRC cells. Conclusion: Our results clearly demonstrate for the first time that resveratrol modulates the TNF-β signaling pathway, induces apoptosis, suppresses NF-κB activation, epithelial-to-mesenchymal-transition (EMT), CSCs formation and chemosensitizes CRC cells to 5-FU in a tumor microenvironment. Keywords: resveratrol; colorectal cancer; cancer stem cells; TNF-β; 5-fluorouracil; alginate culture .1. Introduction
2. Materials and Methods
V体育2025版 - 2.1. Antibodies
2.2. Growth Media, Cytokines and Chemicals
2.3. Cell Lines and Cell Culture
2.4. Alginate Tumor Microenvironment Culture
2.5. Invasion Assay (VSports手机版)
2.6. Immunofluorescence
2.7. Quantification of Apoptosis with DAPI
2.8. Ultrastructural Investigations
"VSports在线直播" 2.9. Western Blot Analysis
V体育平台登录 - 2.10. Statistical Analysis
3. Results
3.1. Resveratrol Chemosensitizes CRC Cells to 5-FU and Suppresses Invasion in TNF-β-, Similar to TNF-α-Induced Pro-Inflammatory Alginate Tumor Microenvironment Cultures (V体育安卓版)
3.2. Resveratrol Suppresses TNF-β-, Similar to TNF-α-Induced Formation of CSCs in Migrated CRC Cells Monolayer Culture as Revealed by Immunofluorescence Microscopy
3.3. Resveratrol Potentiates 5-FU-Mediated Apoptosis in TNF-β-Induced Survival of CRC Cells in Monolayer Cultures
3.4. Resveratrol Suppresses TNF-β- Similar to TNF-α-Enhanced Survival in with 5-FU-Treated CRC Cells by Apoptosis in Alginate Tumor Microenvironment
VSports注册入口 - 3.5. Resveratrol Blocks TNF-β-Induced NF-κB Activation and NF-κB-Dependent Gene Products Involved in Migration, Metastasis and Apoptosis of CRC Cells and Chemosensitizes to 5-FU in Pro-Inflammatory Tumor Microenvironment Cultures
4. Discussion
5. Conclusions
Author Contributions
V体育ios版 - Funding
Acknowledgments
Conflicts of Interest
References
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fedewa, S.A.; Ahnen, D.J.; Meester, R.G.S.; Barzi, A.; Jemal, A. Colorectal cancer statistics, 2017. CA Cancer J. Clin. 2017, 67, 177–193. [Google Scholar] [CrossRef] [PubMed]
- Albini, A.; Cesana, E.; Noonan, D.M. Cancer stem cells and the tumor microenvironment: Soloists or choral singers. Curr. Pharm. Biotechnol. 2011, 12, 171–181. [VSports手机版 - Google Scholar] [CrossRef] [PubMed]
- Gout, S.; Huot, J. Role of cancer microenvironment in metastasis: Focus on colon cancer. Cancer Microenviron. 2008, 1, 69–83. [Google Scholar] [CrossRef] [PubMed]
- Pin, A.L.; Houle, F.; Huot, J. Recent advances in colorectal cancer research: The microenvironment impact. Cancer Microenviron. 2011, 4, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Porta, C.; Larghi, P.; Rimoldi, M.; Totaro, M.G.; Allavena, P.; Mantovani, A.; Sica, A. Cellular and molecular pathways linking inflammation and cancer. Immunobiology 2009, 214, 761–777. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Gehlot, P. Inflammation and cancer: How friendly is the relationship for cancer patients? Curr. Opin. Pharmacol. 2009, 9, 351–369. [Google Scholar] [CrossRef] [PubMed]
- Bharti, A.C.; Aggarwal, B.B. Chemopreventive agents induce suppression of nuclear factor-kappaB leading to chemosensitization. Ann. N. Y. Acad. Sci. 2002, 973, 392–395. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Shishodia, S.; Sandur, S.K.; Pandey, M.K.; Sethi, G. Inflammation and cancer: How hot is the link? Biochem. Pharmacol. 2006, 72, 1605–1621. [Google Scholar] [CrossRef] [PubMed]
- Kunnumakkara, A.B.; Sailo, B.L.; Banik, K.; Harsha, C.; Prasad, S.; Gupta, S.C.; Bharti, A.C.; Aggarwal, B.B. Chronic diseases, inflammation, and spices: How are they linked? J. Transl. Med. 2018, 16, 14. [V体育2025版 - Google Scholar] [CrossRef] [PubMed]
- Balkwill, F. Tumor necrosis factor or tumor promoting factor? Cytokine Growth Factor Rev. 2002, 13, 135–141. [Google Scholar] [CrossRef]
- Balkwill, F.; Mantovani, A. Inflammation and cancer: Back to Virchow? Lancet 2001, 357, 539–545. [Google Scholar] [CrossRef]
- Balkwill, F. Tumour necrosis factor and cancer. Nat. Rev. Cancer 2009, 9, 361–371. [VSports在线直播 - Google Scholar] [CrossRef] [PubMed]
- Lau, T.S.; Chung, T.K.; Cheung, T.H.; Chan, L.K.; Cheung, L.W.; Yim, S.F.; Siu, N.S.; Lo, K.W.; Yu, M.M.; Kulbe, H.; et al. Cancer cell-derived lymphotoxin mediates reciprocal tumour-stromal interactions in human ovarian cancer by inducing CXCL11 in fibroblasts. J. Pathol. 2014, 232, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Schneider, K.; Potter, K.G.; Ware, C.F. Lymphotoxin and LIGHT signaling pathways and target genes. Immunol. Rev. 2004, 202, 49–66. [VSports在线直播 - Google Scholar] [CrossRef] [PubMed]
- Dejardin, E.; Droin, N.M.; Delhase, M.; Haas, E.; Cao, Y.; Makris, C.; Li, Z.W.; Karin, M.; Ware, C.F.; Green, D.R. The lymphotoxin-beta receptor induces different patterns of gene expression via two NF-kappaB pathways. Immunity 2002, 17, 525–535. [Google Scholar] [CrossRef]
- Muller, J.R.; Siebenlist, U. Lymphotoxin beta receptor induces sequential activation of distinct NF-kappa B factors via separate signaling pathways. J. Boil. Chem. 2003, 278, 12006–12012. [Google Scholar] [CrossRef] [PubMed]
- Cupedo, T.; Mebius, R.E. Cellular Interactions in Lymph Node Development. J. Immunol. 2005, 174, 21–25. ["VSports最新版本" Google Scholar] [CrossRef] [PubMed]
- Etemadi, N.; Holien, J.K.; Chau, D.; Dewson, G.; Murphy, J.M.; Alexander, W.S.; Parker, M.W.; Silke, J.; Nachbur, U. Lymphotoxin alpha induces apoptosis, necroptosis and inflammatory signals with the same potency as tumour necrosis factor. FEBS J. 2013, 280, 5283–5297. [Google Scholar] [CrossRef] [PubMed]
- Munro, M.J.; Wickremesekera, S.K.; Peng, L.; Tan, S.T.; Itinteang, T. Cancer stem cells in colorectal cancer: A review. J. Clin. Pathol. 2017, 71, 110–116. ["VSports在线直播" Google Scholar] [CrossRef] [PubMed]
- Kreso, A.; Dick, J.E. Evolution of the cancer stem cell model. Cell Stem Cell 2014, 14, 275–291. [Google Scholar] [CrossRef] [PubMed]
- Ricci-Vitiani, L.; Fabrizi, E.; Palio, E.; De Maria, R. Colon cancer stem cells. J. Mol. Med. 2009, 87, 1097–1104. ["VSports" Google Scholar] [CrossRef] [PubMed]
- Jiao, Z.Y.; Cao, H.T.; Li, Y.M. Possible Role of Cancer Stem Cells in Colorectal Cancer Metastasizing to the Liver. Curr. Stem Cell Res. Ther. 2016, 11, 440–443. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, D.; Dick, J.E. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat. Med. 1997, 3, 730–737. [Google Scholar] [CrossRef] [PubMed]
- Schiavoni, G.; Gabriele, L.; Mattei, F. The tumor microenvironment: A pitch for multiple players. Front. Oncol. 2013, 3, 90. [Google Scholar] [CrossRef] [PubMed]
- Boral, D.; Nie, D. Cancer stem cells and niche mircoenvironments. Front. Biosci. 2012, 4, 2502–2514. ["V体育ios版" Google Scholar]
- Iqbal, A.; George, T.J. Randomized Clinical Trials in Colon and Rectal Cancer. Surg. Oncol. Clin. N. Am. 2017, 26, 689–704. [Google Scholar] [CrossRef] [PubMed]
- Sha, A.; Abadi, S.; Gill, S. Utilization of capecitabine plus oxaliplatin and 5-fluorouracil/folinic acid plus oxaliplatin in the adjuvant treatment of stage IIB and stage III colon cancer: A multi-centre, retrospective, chart review study. J. Oncol. Pharm. Pract. 2017. ["VSports" Google Scholar] [CrossRef] [PubMed]
- Dick, J.E. Stem cell concepts renew cancer research. Blood 2008, 112, 4793–4807. [Google Scholar] [CrossRef] [PubMed]
- Bansal, N.; Banerjee, D. Tumor initiating cells. Curr. Pharm. Biotechnol. 2009, 10, 192–196. [Google Scholar] [CrossRef] [PubMed]
- Toden, S.; Tran, H.M.; Tovar-Camargo, O.A.; Okugawa, Y.; Goel, A. Epigallocatechin-3-gallate targets cancer stem-like cells and enhances 5-fluorouracil chemosensitivity in colorectal cancer. Oncotarget 2016, 7, 16158–16171. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Kanwar, S.S.; Patel, B.B.; Nautiyal, J.; Sarkar, F.H.; Majumdar, A.P. Elimination of Colon Cancer Stem-Like Cells by the Combination of Curcumin and FOLFOX. Transl. Oncol. 2009, 2, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Shakibaei, M.; Kraehe, P.; Popper, B.; Shayan, P.; Goel, A.; Buhrmann, C. Curcumin potentiates antitumor activity of 5-fluorouracil in a 3D alginate tumor microenvironment of colorectal cancer. BMC Cancer 2015, 15, 250. [Google Scholar (VSports在线直播)] [CrossRef] [PubMed]
- Toden, S.; Okugawa, Y.; Jascur, T.; Wodarz, D.; Komarova, N.L.; Buhrmann, C.; Shakibaei, M.; Boland, C.R.; Goel, A. Curcumin mediates chemosensitization to 5-fluorouracil through miRNA-induced suppression of epithelial-to-mesenchymal transition in chemoresistant colorectal cancer. Carcinogenesis 2015, 36, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Buhrmann, C.; Kraehe, P.; Lueders, C.; Shayan, P.; Goel, A.; Shakibaei, M. Curcumin suppresses crosstalk between colon cancer stem cells and stromal fibroblasts in the tumor microenvironment: Potential role of EMT. PLoS ONE 2014, 9, e107514. [Google Scholar] [CrossRef] [PubMed]
- Shakibaei, M.; Harikumar, K.B.; Aggarwal, B.B. Resveratrol addiction: To die or not to die. Mol. Nutr. Food Res. 2009, 53, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Daniel, O.; Meier, M.S.; Schlatter, J.; Frischknecht, P. Selected phenolic compounds in cultivated plants: Ecologic functions, health implications, and modulation by pesticides. Environ. Health Perspect. 1999, 107 (Suppl. 1), 109–114. [Google Scholar] [CrossRef] [PubMed]
- Khanna, D.; Sethi, G.; Ahn, K.S.; Pandey, M.K.; Kunnumakkara, A.B.; Sung, B.; Aggarwal, A.; Aggarwal, B.B. Natural products as a gold mine for arthritis treatment. Curr. Opin. Pharmacol. 2007, 7, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.Y.; Phoo, M.S.; Clement, M.V.; Pervaiz, S.; Lee, S.C. Resveratrol displays converse dose-related effects on 5-fluorouracil-evoked colon cancer cell apoptosis: The roles of caspase-6 and p53. Cancer Boil. Ther. 2008, 7, 1305–1312. [Google Scholar] [CrossRef]
- Aires, V.; Limagne, E.; Cotte, A.K.; Latruffe, N.; Ghiringhelli, F.; Delmas, D. Resveratrol metabolites inhibit human metastatic colon cancer cells progression and synergize with chemotherapeutic drugs to induce cell death. Mol. Nutr. Food Res. 2013, 57, 1170–1181. ["VSports最新版本" Google Scholar] [CrossRef] [PubMed]
- Buhrmann, C.; Shayan, P.; Goel, A.; Shakibaei, M. Resveratrol Regulates Colorectal Cancer Cell Invasion by Modulation of Focal Adhesion Molecules. Nutrients 2017, 9, 1073. [Google Scholar] [CrossRef] [PubMed]
- Temraz, S.; Mukherji, D.; Shamseddine, A. Potential targets for colorectal cancer prevention. Int. J. Mol. Sci. 2013, 14, 17279–17303. [Google Scholar (VSports手机版)] [CrossRef] [PubMed]
- Cal, C.; Garban, H.; Jazirehi, A.; Yeh, C.; Mizutani, Y.; Bonavida, B. Resveratrol and cancer: Chemoprevention, apoptosis, and chemo-immunosensitizing activities. Curr. Med. Chem. 2003, 3, 77–93. [Google Scholar]
- Buhrmann, C.; Shayan, P.; Kraehe, P.; Popper, B.; Goel, A.; Shakibaei, M. Resveratrol induces chemosensitization to 5-fluorouracil through up-regulation of intercellular junctions, Epithelial-to-mesenchymal transition and apoptosis in colorectal cancer. Biochem. Pharm. 2015, 98, 51–68. [Google Scholar] [CrossRef] [PubMed]
- Bringman, T.S.; Aggarwal, B.B. Monoclonal antibodies to human tumor necrosis factors alpha and beta: Application for affinity purification, immunoassays, and as structural probes. Hybridoma 1987, 6, 489–507. [Google Scholar] [CrossRef] [PubMed]
- Shakibaei, M.; John, T.; De Souza, P.; Rahmanzadeh, R.; Merker, H.J. Signal transduction by beta1 integrin receptors in human chondrocytes in vitro: Collaboration with the insulin-like growth factor-I receptor. Biochem. J. 1999, 342 Pt 3, 615–623. ["VSports" Google Scholar] [CrossRef] [PubMed]
- Vermeulen, L.; De Sousa, E.M.F.; van der Heijden, M.; Cameron, K.; de Jong, J.H.; Borovski, T.; Tuynman, J.B.; Todaro, M.; Merz, C.; Rodermond, H.; et al. Wnt activity defines colon cancer stem cells and is regulated by the microenvironment. Nat. Cell Boil. 2010, 12, 468–476. ["VSports手机版" Google Scholar] [CrossRef] [PubMed]
- Powell, D.W.; Mifflin, R.C.; Valentich, J.D.; Crowe, S.E.; Saada, J.I.; West, A.B. Myofibroblasts. II. Intestinal subepithelial myofibroblasts. Am. J. Physiol. 1999, 277, C183–C201. [V体育2025版 - Google Scholar] [CrossRef] [PubMed]
- Balkwill, F.; Mantovani, A. Cancer and inflammation: Implications for pharmacology and therapeutics. Clin. Pharmacol. Ther. 2010, 87, 401–406. [Google Scholar (V体育平台登录)] [CrossRef] [PubMed]
- Hanahan, D.; Coussens, L.M. Accessories to the crime: Functions of cells recruited to the tumor microenvironment. Cancer Cell 2012, 21, 309–322. [Google Scholar] [CrossRef] [PubMed]
- Mine, N.; Yamamoto, S.; Saito, N.; Sato, T.; Sakakibara, K.; Kufe, D.W.; VonHoff, D.D.; Kawabe, T. CBP501 suppresses macrophage induced cancer stem cell like features and metastases. Oncotarget 2017, 8, 64015–64031. [Google Scholar] [CrossRef] [PubMed]
- Sethi, G.; Sung, B.; Aggarwal, B.B. TNF: A master switch for inflammation to cancer. Front. Biosci. 2008, 13, 5094–5107. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B. Nuclear factor-kappaB: The enemy within. Cancer Cell 2004, 6, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Thiery, J.P. Epithelial-mesenchymal transitions in tumour progression. Nat. Rev. Cancer 2002, 2, 442–454. [Google Scholar] [CrossRef] [PubMed]
- Bolos, V.; Peinado, H.; Perez-Moreno, M.A.; Fraga, M.F.; Esteller, M.; Cano, A. The transcription factor Slug represses E-cadherin expression and induces epithelial to mesenchymal transitions: A comparison with Snail and E47 repressors. J. Cell Sci. 2003, 116 Pt 3, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Bhardwaj, A.; Aggarwal, R.S.; Seeram, N.P.; Shishodia, S.; Takada, Y. Role of resveratrol in prevention and therapy of cancer: Preclinical and clinical studies. Anticancer Res. 2004, 24, 2783–2840. [Google Scholar] [PubMed]
- Lu, H.; Ouyang, W.; Huang, C. Inflammation, a key event in cancer development. Mol. Cancer Res. 2006, 4, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Karin, M. Nuclear factor-kappaB in cancer development and progression. Nature 2006, 441, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Sounni, N.E.; Noel, A. Targeting the tumor microenvironment for cancer therapy. Clin. Chem. 2013, 59, 85–93. ["VSports最新版本" Google Scholar] [CrossRef] [PubMed]
- Tlsty, T.D. Stromal cells can contribute oncogenic signals. Semin. Cancer Boil. 2001, 11, 97–104. [Google Scholar (VSports最新版本)] [CrossRef] [PubMed]
- Liotta, L.A.; Kohn, E.C. The microenvironment of the tumour-host interface. Nature 2001, 411, 375–379. [Google Scholar] [CrossRef] [PubMed]
- Oliver, A.J.; Lau, P.K.H.; Unsworth, A.S.; Loi, S.; Darcy, P.K.; Kershaw, M.H.; Slaney, C.Y. Tissue-Dependent Tumor Microenvironments and Their Impact on Immunotherapy Responses. Front. Immunol. 2018, 9, 70. [Google Scholar] [CrossRef] [PubMed]
- Balkwill, F.; Charles, K.A.; Mantovani, A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell 2005, 7, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Raposo, T.P.; Beirao, B.C.; Pang, L.Y.; Queiroga, F.L.; Argyle, D.J. Inflammation and cancer: Till death tears them apart. Vet. J. 2015, 205, 161–174. ["V体育ios版" Google Scholar] [CrossRef] [PubMed]
- Rakoff-Nahoum, S. Why cancer and inflammation? Yale J. Boil. Med. 2006, 79, 123–130. [V体育官网入口 - Google Scholar]
- Dalerba, P.; Dylla, S.J.; Park, I.K.; Liu, R.; Wang, X.; Cho, R.W.; Hoey, T.; Gurney, A.; Huang, E.H.; Simeone, D.M.; et al. Phenotypic characterization of human colorectal cancer stem cells. Proc. Natl. Acad. Sci. USA 2007, 104, 10158–10163. [Google Scholar] [CrossRef] [PubMed]
- Chu, P.; Clanton, D.J.; Snipas, T.S.; Lee, J.; Mitchell, E.; Nguyen, M.L.; Hare, E.; Peach, R.J. Characterization of a subpopulation of colon cancer cells with stem cell-like properties. Int. J. Cancer 2009, 124, 1312–1321. ["V体育平台登录" Google Scholar] [CrossRef] [PubMed]
- Boman, B.M.; Wicha, M.S. Cancer stem cells: A step toward the cure. J. Clin. Oncol. 2008, 26, 2795–2799. [VSports最新版本 - Google Scholar] [CrossRef] [PubMed]
- Dalerba, P.; Clarke, M.F. Cancer stem cells and tumor metastasis: First steps into uncharted territory. Cell Stem Cell 2007, 1, 241–242. [Google Scholar] [CrossRef] [PubMed]
- Jordan, C.T.; Guzman, M.L.; Noble, M. Cancer stem cells. N. Engl. J. Med. 2006, 355, 1253–1261. [Google Scholar (V体育2025版)] [CrossRef] [PubMed]
- De Carlo, F.; Witte, T.R.; Hardman, W.E.; Claudio, P.P. Omega-3 eicosapentaenoic acid decreases CD133 colon cancer stem-like cell marker expression while increasing sensitivity to chemotherapy. PLoS ONE 2013, 8, e69760. [Google Scholar] [CrossRef] [PubMed]
- Todaro, M.; Alea, M.P.; Di Stefano, A.B.; Cammareri, P.; Vermeulen, L.; Iovino, F.; Tripodo, C.; Russo, A.; Gulotta, G.; Medema, J.P.; et al. Colon cancer stem cells dictate tumor growth and resist cell death by production of interleukin-4. Cell Stem Cell 2007, 1, 389–402. [Google Scholar] [CrossRef] [PubMed]
- Todaro, M.; Francipane, M.G.; Medema, J.P.; Stassi, G. Colon cancer stem cells: Promise of targeted therapy. Gastroenterology 2010, 138, 2151–2162. [Google Scholar] [CrossRef] [PubMed]
- Dean, M.; Fojo, T.; Bates, S. Tumour stem cells and drug resistance. Nat. Rev. Cancer 2005, 5, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.L.; Sun, Z.J.; Yu, L.; Meng, K.W.; Qin, X.L.; Pan, C.E. Effect of resveratrol and in combination with 5-FU on murine liver cancer. World J. Gastroenterol. 2004, 10, 3048–3052. [Google Scholar] [CrossRef] [PubMed]
- Karin, M.; Greten, F.R. NF-kappaB: Linking inflammation and immunity to cancer development and progression. Nat. Rev. Immunol. 2005, 5, 749–759. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Takada, Y.; Boriek, A.M.; Aggarwal, B.B. Nuclear factor-kappaB: Its role in health and disease. J. Mol. Med. 2004, 82, 434–448. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.C.; Kim, J.H.; Prasad, S.; Aggarwal, B.B. Regulation of survival, proliferation, invasion, angiogenesis, and metastasis of tumor cells through modulation of inflammatory pathways by nutraceuticals. Cancer Metastasis Rev. 2010, 29, 405–434. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Shishodia, S. Molecular targets of dietary agents for prevention and therapy of cancer. Biochem. Pharmacol. 2006, 71, 1397–1421. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.F.; Mu, Y.; Greene, W.C. Acetylation of RelA at discrete sites regulates distinct nuclear functions of NF-kappaB. EMBO J. 2002, 21, 6539–6548. [Google Scholar] [CrossRef] [PubMed]
- Buhrmann, C.; Shayan, P.; Popper, B.; Goel, A.; Shakibaei, M. Sirt1 Is Required for Resveratrol-Mediated Chemopreventive Effects in Colorectal Cancer Cells. Nutrients 2016, 8, 145. [VSports最新版本 - Google Scholar] [CrossRef] [PubMed]
- Milne, J.C.; Lambert, P.D.; Schenk, S.; Carney, D.P.; Smith, J.J.; Gagne, D.J.; Jin, L.; Boss, O.; Perni, R.B.; Vu, C.B.; et al. Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature 2007, 450, 712–716. [Google Scholar] [CrossRef] [PubMed]
- Knight, C.M.; Gutierrez-Juarez, R.; Lam, T.K.; Arrieta-Cruz, I.; Huang, L.; Schwartz, G.; Barzilai, N.; Rossetti, L. Mediobasal hypothalamic SIRT1 is essential for resveratrol's effects on insulin action in rats. Diabetes 2011, 60, 2691–2700. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, S.; Loitsch, S.M.; Rau, O.; von Knethen, A.; Brune, B.; Schubert-Zsilavecz, M.; Stein, J.M. Peroxisome proliferator-activated receptor gamma as a molecular target of resveratrol-induced modulation of polyamine metabolism. Cancer Res. 2006, 66, 7348–7354. [Google Scholar] [CrossRef] [PubMed]
- Polyak, K.; Weinberg, R.A. Transitions between epithelial and mesenchymal states: Acquisition of malignant and stem cell traits. Nat. Rev. Cancer 2009, 9, 265–273. [Google Scholar (VSports最新版本)] [CrossRef] [PubMed]
- Gunasinghe, N.P.; Wells, A.; Thompson, E.W.; Hugo, H.J. Mesenchymal-epithelial transition (MET) as a mechanism for metastatic colonisation in breast cancer. Cancer Metastasis Rev. 2012, 31, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Bonnomet, A.; Syne, L.; Brysse, A.; Feyereisen, E.; Thompson, E.W.; Noel, A.; Foidart, J.M.; Birembaut, P.; Polette, M.; Gilles, C. A dynamic in vivo model of epithelial-to-mesenchymal transitions in circulating tumor cells and metastases of breast cancer. Oncogene 2012, 31, 3741–3753. [Google Scholar] [CrossRef] [PubMed]
- Dimanche-Boitrel, M.T.; Vakaet, L., Jr.; Pujuguet, P.; Chauffert, B.; Martin, M.S.; Hammann, A.; Van Roy, F.; Mareel, M.; Martin, F. In vivo and in vitro invasiveness of a rat colon-cancer cell line maintaining E-cadherin expression: An enhancing role of tumor-associated myofibroblasts. Int. J. Cancer 1994, 56, 512–521. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, G.; Wang, Z.; Fang, X.; Liu, J.; Yang, C.J. Molecular signaling of the epithelial to mesenchymal transition in generating and maintaining cancer stem cells. Cell. Mol. Life Sci. 2010, 67, 2605–2618. [Google Scholar (VSports在线直播)] [CrossRef] [PubMed]






© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buhrmann, C.; Yazdi, M.; Popper, B.; Shayan, P.; Goel, A.; Aggarwal, B.B.; Shakibaei, M. Resveratrol Chemosensitizes TNF-β-Induced Survival of 5-FU-Treated Colorectal Cancer Cells. Nutrients 2018, 10, 888. https://doi.org/10.3390/nu10070888
Buhrmann C, Yazdi M, Popper B, Shayan P, Goel A, Aggarwal BB, Shakibaei M. Resveratrol Chemosensitizes TNF-β-Induced Survival of 5-FU-Treated Colorectal Cancer Cells. Nutrients. 2018; 10(7):888. https://doi.org/10.3390/nu10070888
Chicago/Turabian StyleBuhrmann, Constanze, Mina Yazdi, Bastian Popper, Parviz Shayan, Ajay Goel, Bharat B. Aggarwal, and Mehdi Shakibaei. 2018. "Resveratrol Chemosensitizes TNF-β-Induced Survival of 5-FU-Treated Colorectal Cancer Cells" Nutrients 10, no. 7: 888. https://doi.org/10.3390/nu10070888
APA StyleBuhrmann, C., Yazdi, M., Popper, B., Shayan, P., Goel, A., Aggarwal, B. B., & Shakibaei, M. (2018). Resveratrol Chemosensitizes TNF-β-Induced Survival of 5-FU-Treated Colorectal Cancer Cells. Nutrients, 10(7), 888. https://doi.org/10.3390/nu10070888